45 how to cite fda drug label
How do I cite a product label or packaging information? When you discuss or quote from a product label or packaging information, you do not generally need to create a works-cited-list entry. You can provide all necessary details in your prose: Food-and-beverage packaging frequently touts a product's healthy qualities. For example, a package produced in 2013 for Yogi Green Tea Super Antioxidant—a ... Reference Listed Drug, RLD, ANDA, Generic drug, FDA - Freyr Solutions A Reference Listed Drug (RLD), as goes by its innate meaning, is an FDA approved drug product which can be referred to by a generic drug manufacturer while filing an Abbreviated New Drug Application (ANDA). An RLD is basically useful to establish bioequivalence of the product with that of an already approved one. When a generical manufacturer ...
PDF Citing FDA Approval Letters - Jane Ganter Citing FDA Approval Letters Citing electronic sources can tax the capacity of an obedient author to conform to the style manual. But I rely on, and take comfort from, passages in APA's Publication Manual like this one (p. 269). The variety of material available on the Web, and the variety of ways in which it is structured

How to cite fda drug label
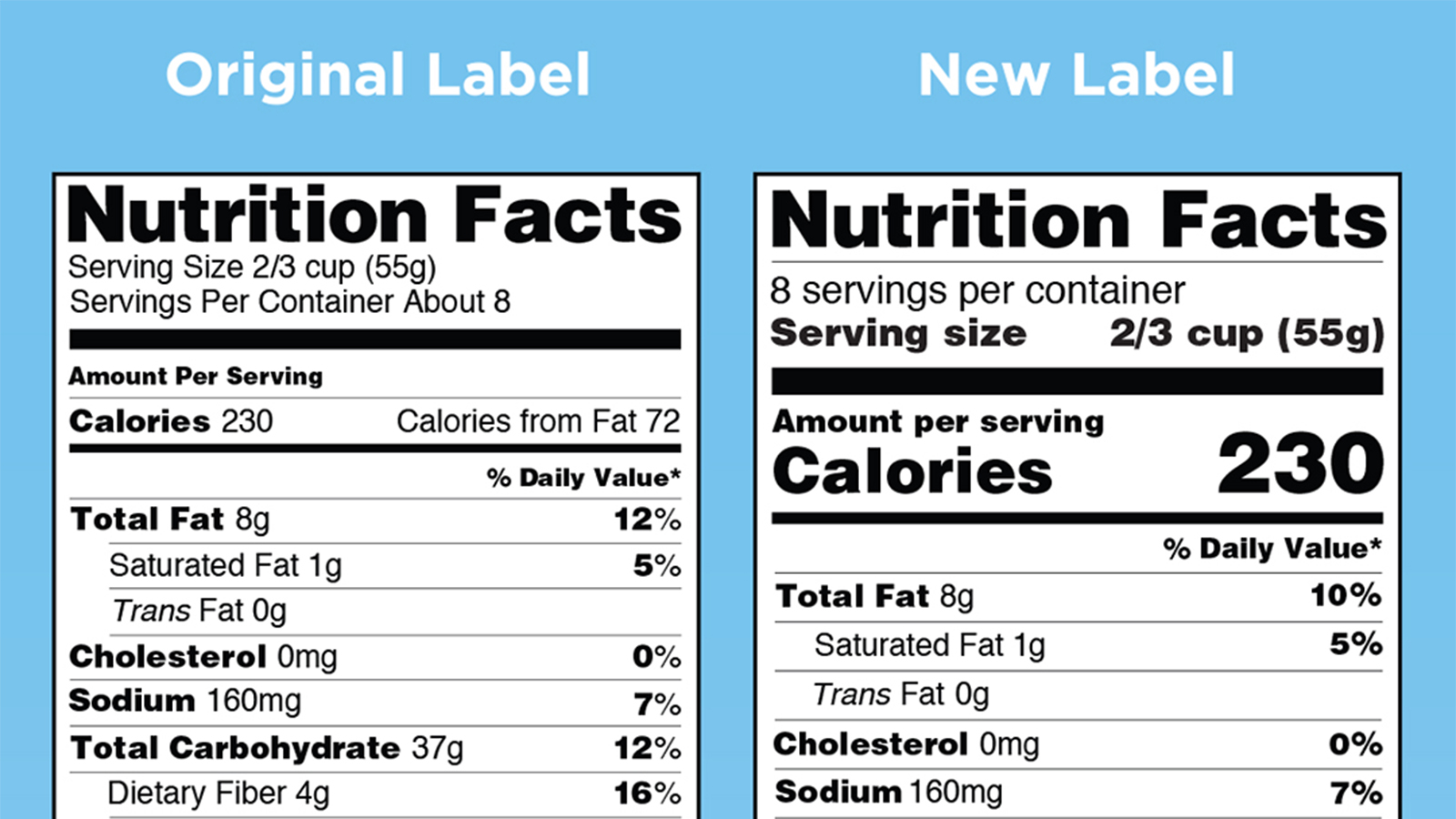
CFR - Code of Federal Regulations Title 21 - Food and Drug … 20.07.2022 · (B) Quantitative information comparing the level of the sugar in the product per labeled serving with that of the reference food that it replaces (e.g., "Sugar content has been lowered from 8 g to 6 g per serving.") is declared adjacent to the most prominent claim or to the nutrition label, except that if the nutrition label is on the information panel, the quantitative … Q. How do I cite a drug package insert? - LibAnswers EndNote Formatting. In EndNote, you can manually create a package insert citation by going to References and then New Reference. Choose Journal Article for the reference type. Enter the citation into the Title field within the EndNote citation you are creating. Save the citation upon closing, and then use it normally as any other EndNote reference. How Do I Use Prescription Drug Labeling | FDA FDA-Approved Patient Labeling Patient labeling may be physically attached or provided separately from the USPI and contains information in lay language that can help patients use a drug...
How to cite fda drug label. Implementing the PLR Content and Format Requirements - ProPharma Group In 2013, the FDA released a guidance entitled "Labeling for Human Prescription Drug and Biological Products - Implementing the PLR Content and Format Requirements."" This guidance was finalized after taking industry comments into consideration, and was issued to assist Sponsors in complying with content and format requirements for labeling drug and biological products under the ... A Prescription for Success: How to Cite Product Information in ... The distributor is listed on the insert as Shionogi Pharma, so we'll put that in the author position (in accordance with our principle of "cite what you see"). When was it made? The date on the insert is 2010, so that goes in the date position. What is the document called? CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (2) Section 403(g)(2) of the act (requiring the label of a food which purports to be or is represented as one for which a definition and standard of identity has been prescribed to bear the name of the food specified in the definition and standard and, insofar as may be required by the regulation establishing the standard the common names of ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
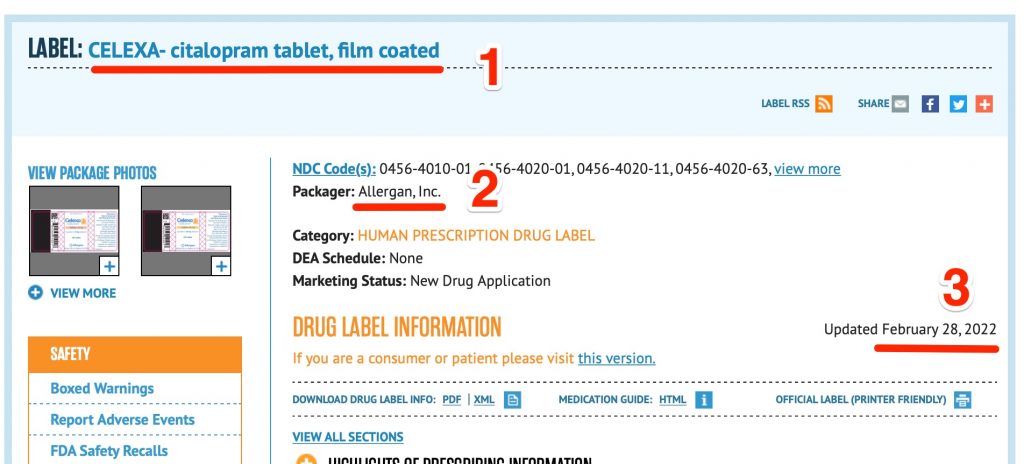
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... APA Citation Style Guide (6th Ed.): Government Publication APA style for citing Congressional publications is based on the latest edition of The Bluebook: A Uniform System of Citation. (p. 216) For information about citing these sources in your reference list, see the Westfield State University page on Citing Legal Materials in APA Style. Package Inserts - Vancouver Citation Style Guide 14 Aug 2022 — Drug name [package insert]. Place of publication: Manufacturer; publication year. Example of a Package Insert Citation. CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · The requirements in this section apply to the label or labeling of dietary supplements where the dietary supplement bears a statement that is provided for by section 403(r)(6) of the Federal Food, Drug, and Cosmetic Act (the act), and the manufacturer, packer, or distributor wishes to take advantage of the exemption to section 201(g)(1)(C) of ...
Package Insert - Reference Guide for Pharmacy Students Citation format based on AMA for College of Pharmacy students. Referencing Guide - College of Pharmacy; Journal Article (print or electronic) ... Package Insert; Internet Sites; Drug Databases; Other; Package Insert Medication Name. Package insert. Manufacturer's Name; Year. (registered or updated, whichever is most recent) Example: Byetta ... Reference List - AMA Style (11th ed): Citing Your Sources 7 Oct 2022 — Name of drug. Type of material. Company Name; year of publication. To indicate online access, add the accessed date and URL. Examples: Lamasil. FDALabel: Full-Text Search of Drug Product Labeling | FDA Because the SPL documents on FDALabel may not be identical to the most recent FDA-approved labeling, visit the following sites for the most current FDA-approved labeling: Drugs@FDA for... Referencing/Citing Drugs.com Subscribe to Drugs.com newsletters for the latest medication news, new drug approvals, alerts and updates. Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products.
Citing and referencing: Drug information sources - Monash University Citing and referencing: Drug information sources Drug information sources Before using this guide check with your faculty, school or department for their specific referencing guidelines Formularies (print) Entry from formulary (print) Drug monographs (electronic) Drug interaction (electronic) Package insert (Leaflet supplied by manufacturer)
How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Provide the name of the drug and title of the package insert in italics. Type the full name of the drug followed by a colon. Then type the title of the package insert as stated at the top of the insert. Type the title in sentence-case, capitalizing only the first word and any proper nouns. Place a period at the end of the title. [4]
FDALabel - U.S. Food and Drug Administration Labeling, Product and Ingredient Identifiers. Application Number for ANDA, BLA, or NDA: 3 to 6 digits (e.g., 077844, 125118, 020977) Unique Ingredient Identifier (UNII): To search for active ingredients, inactive ingredients or both, type in alphanumeric code (s) (e.g., J220T4J9Q2)
PCOM Library LibGuides Citation Guide: ICMJE Package Inserts Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers does not contain specific information about how to cite package insert. Please use the following format. Drug name [package insert]. Place of publication: Manufacturer's name; Year of publication. Example. Albuterol [package insert]. West Roxbury, MA: Armstrong ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) Prescription drug labeling described in § 201.100 (d) must contain the specific information required under § 201.57 (a), (b), and (c) under the following headings and subheadings and in the...
DailyMed The National Library of Medicine (NLM)'s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary ...
APA Citation Style, 7th edition: Government Publication (U. S. Food and Drug Administration, 2004) In-Text Citation (Quotation): (U. S. Food and Drug Administration, 2004, p. 8) References: U. S. Food and Drug Administration/Center for Drug Evaluation and Research. (2004). Worsening depression and suicidality in patients being treated with antidepressant medications: FDA public health advisory. Author.
LibGuides: AMA Citation Guide: Drug Info & Medical Resources Use the drug name or page title for the information you are citing and the subpage with a comma (example 2) List the section of AccessPharmacy or AccessMedicine you are citing, like "Drugs" To cite books from AccessPharmacy and AccessMedicine, follow the instructions on the AccessMedicine guide citation page ClinicalTrials.gov 3.
Q. How do I cite a prescription insert in APA 7 format? There are three main components you will need for all three citation options: Who is responsible for the content of the package insert? The distributor is listed on the insert as Shionogi Pharma, so we'll put that in the author position (in accordance with our principle of "cite what you see"). When was it made?
FDA Label Search-Application Number - Food and Drug Administration Search by Application Number or Regulatory Citation: For application numbers, type the 6 digit application number, including the leading zero. For citations, type in "part" and at least a portion...
Guidance for Industry and FDA Staff - U.S. Food and Drug ... 1. Introduction. FDA has developed this guidance document to assist industry in preparing premarket notification submissions (510(k)) for menstrual tampons and pads that are subject to 510(k ...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (i) If the label, labeling, or advertising of a food makes any direct or indirect representations with respect to the primary recognizable flavor(s), by word, vignette, e.g., depiction of a fruit, or other means, or if for any other reason the manufacturer or distributor of a food wishes to designate the type of flavor in the food other than ...
How to Cite a Product Label in APA Format - Pen and the Pad When citing the product label reference in the text of your work, use an in-text citation. This is parenthetical text that includes the manufacturer's name and the year of manufacture: (General Mills, 2015) (The Quaker Oats Company, 2015) Need help with a citation? Try our citation generator.
Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 09, 2016 · Copies are available from the Office of Food Additive Safety (HFS-200), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-1200, or may be examined at the Food and Drug Administration's Main Library, 10903 New Hampshire Ave., Bldg. 2, Third Floor, Silver Spring, MD 20993 ...
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical devices.
NEOMED Library: Citing Resources: AMA Citation To cite in text using AMA, you will use superscripts just behind the idea you are citing. Superscript numbers are placed outside periods and commas, and inside colons and semicolons. To cite more than 1 article with the same idea, use commas to separate two references 1,2. Example:
How do I cite a drug label? - Ask HSL Jul 27, 2017 view. This answer is handled in our Citing Special Sources guide. In AMA: Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceuticals Corp; 1993. In APA: Sandoz Pharmaceuticals Corp. (1993). Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceuticals Corp.
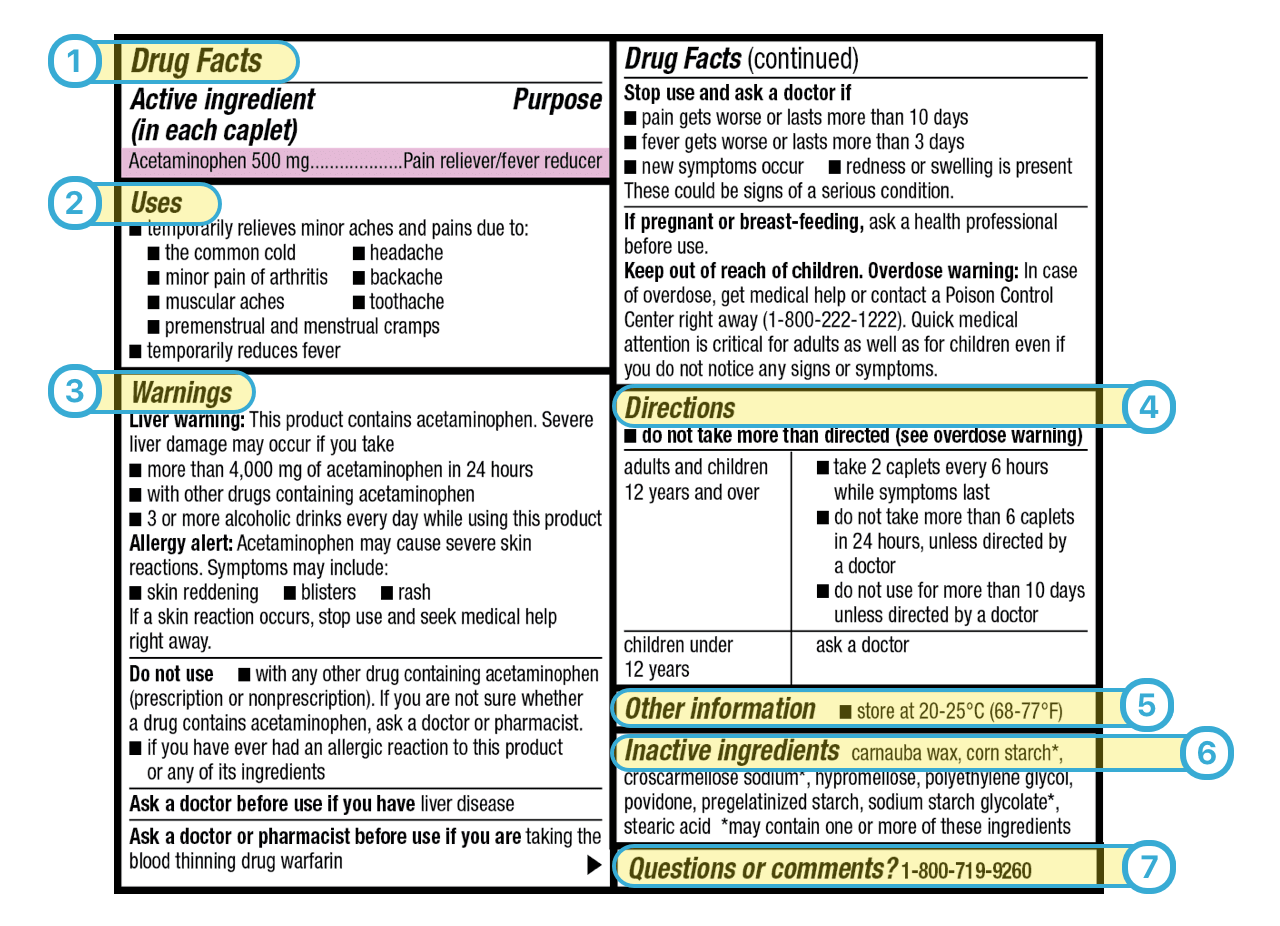
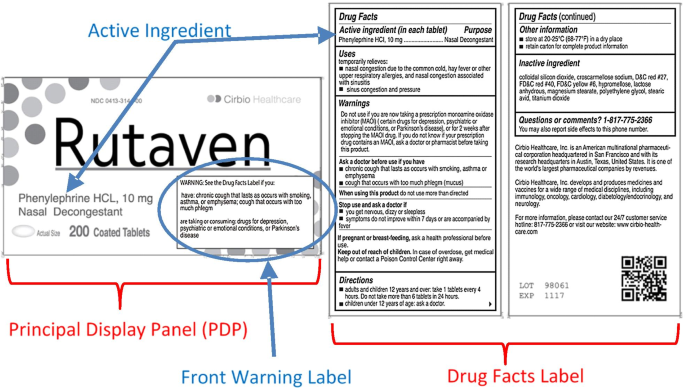
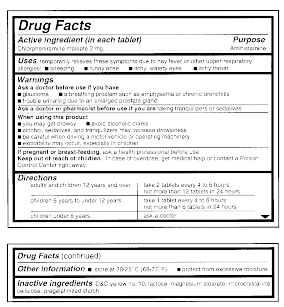
Pharmaceutical Labeling: Requirements & Guidelines - CTM Labeling Systems All drug products must be registered with the FDA, have a National Drug Code (NDC), and have that three-section NDC code printed on the front of the label. (You can read more about the NDC here.) Breaking Down the Drug Facts Table To clarify drug information, the FDA requires that all pharmaceutical product labels include a "Drug Facts" table.
Federal Register :: Revising Abbreviated New Drug Application Labeling ... FDA regulations provide examples of permissible differences in labeling that may result when a proposed generic drug and the RLD are "produced or distributed by different manufacturers," including the omission of an indication or other aspect of labeling protected by patent or exclusivity and "labeling revisions made to comply with ...
E-Cigarettes, Vapes, and other Electronic Nicotine Delivery ... Products marketed for therapeutic purposes (for example, marketed as a product to help people quit smoking) are regulated by FDA’s Center for Drug Evaluation and Research (CDER).
How do I cite / reference product information sheets in ... - FAQS 22 Jun 2020 — In the case of a pharmaceutical insert or information sheet, the author would likely be the distributor or pharmaceutical company, ...
How Do I Use Prescription Drug Labeling | FDA FDA-Approved Patient Labeling Patient labeling may be physically attached or provided separately from the USPI and contains information in lay language that can help patients use a drug...
Q. How do I cite a drug package insert? - LibAnswers EndNote Formatting. In EndNote, you can manually create a package insert citation by going to References and then New Reference. Choose Journal Article for the reference type. Enter the citation into the Title field within the EndNote citation you are creating. Save the citation upon closing, and then use it normally as any other EndNote reference.
CFR - Code of Federal Regulations Title 21 - Food and Drug … 20.07.2022 · (B) Quantitative information comparing the level of the sugar in the product per labeled serving with that of the reference food that it replaces (e.g., "Sugar content has been lowered from 8 g to 6 g per serving.") is declared adjacent to the most prominent claim or to the nutrition label, except that if the nutrition label is on the information panel, the quantitative …


























Post a Comment for "45 how to cite fda drug label"