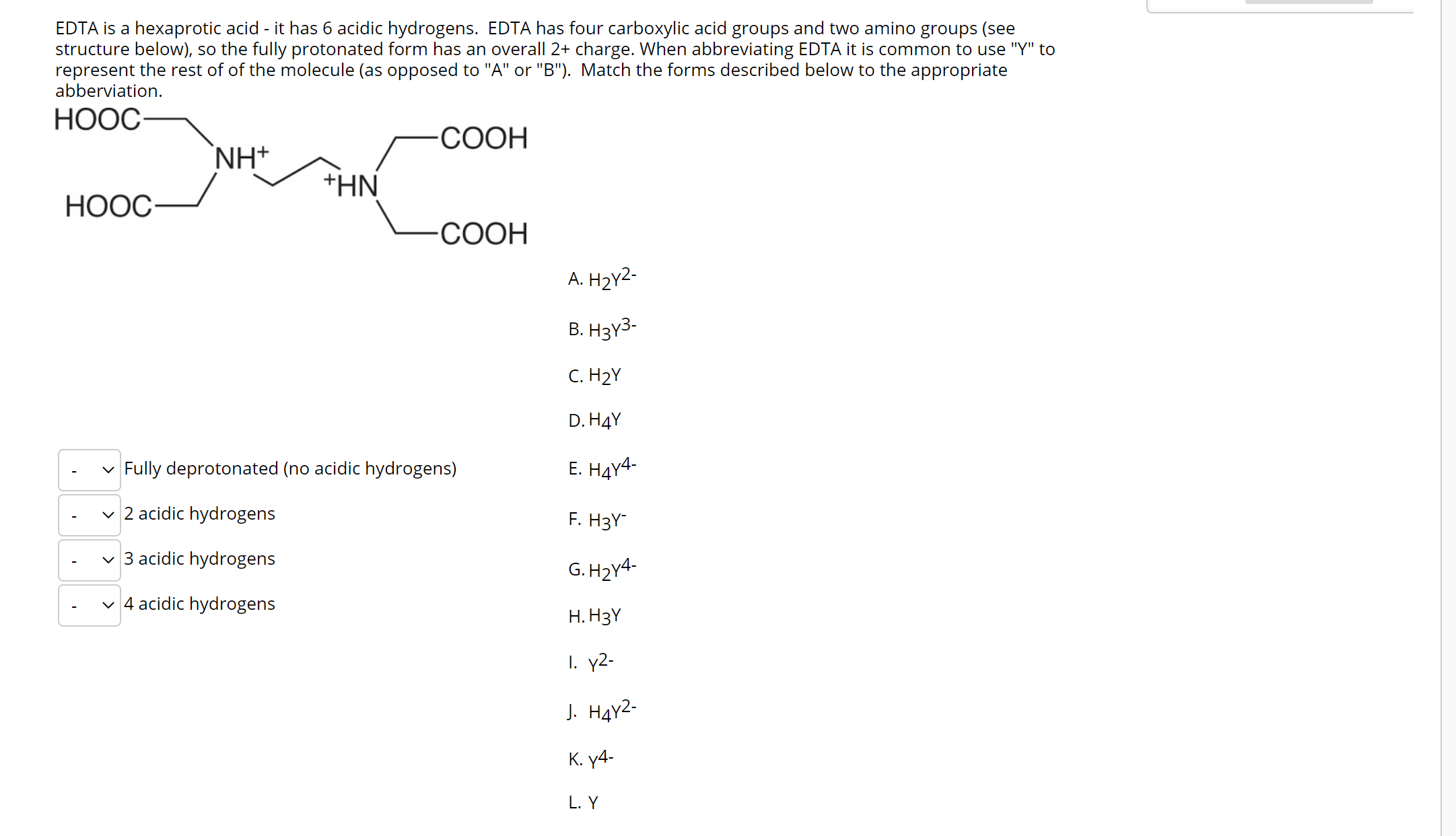
45 h2y acid or base
Solved Identify each reactant and product in this reaction - Chegg Chemistry questions and answers. Identify each reactant and product in this reaction as a Brønsted acid or base. HY + H, Z =HAY + HZ- base base acid base Answer Bank base acid Calculate [H], [C107), and [OH") in an aqueous solution that is 0.145 M in HCIO, (aq) at 25 °C. 130 M Incorrect .130 (CIO) = M Incorrect JOH") - 7.69 x10-14 M Incorrect. H2Y- Acid Or Base - 2 | Haryati Oman Acids are proton donors and bases are proton acceptors h2y acid or base. A is solution containing 6.22 g of an acid h2y per dm3. Label each reactant and product in this reaction as a bronsted acid or base. 1 answer to label each reactant and product in this reaction as a bronsted acid or base.
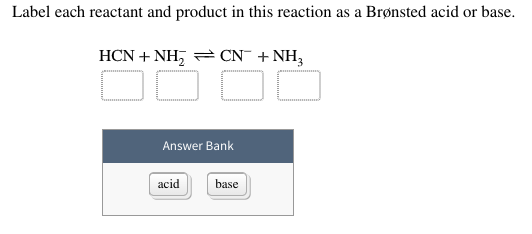
Question: Label each reactant and product in this reaction as a ... - Chegg Science Chemistry Chemistry questions and answers Label each reactant and product in this reaction as a Bronsted acid or base. H2Y- + H2Z- <===> H3Y + HZ2- This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

H2y acid or base
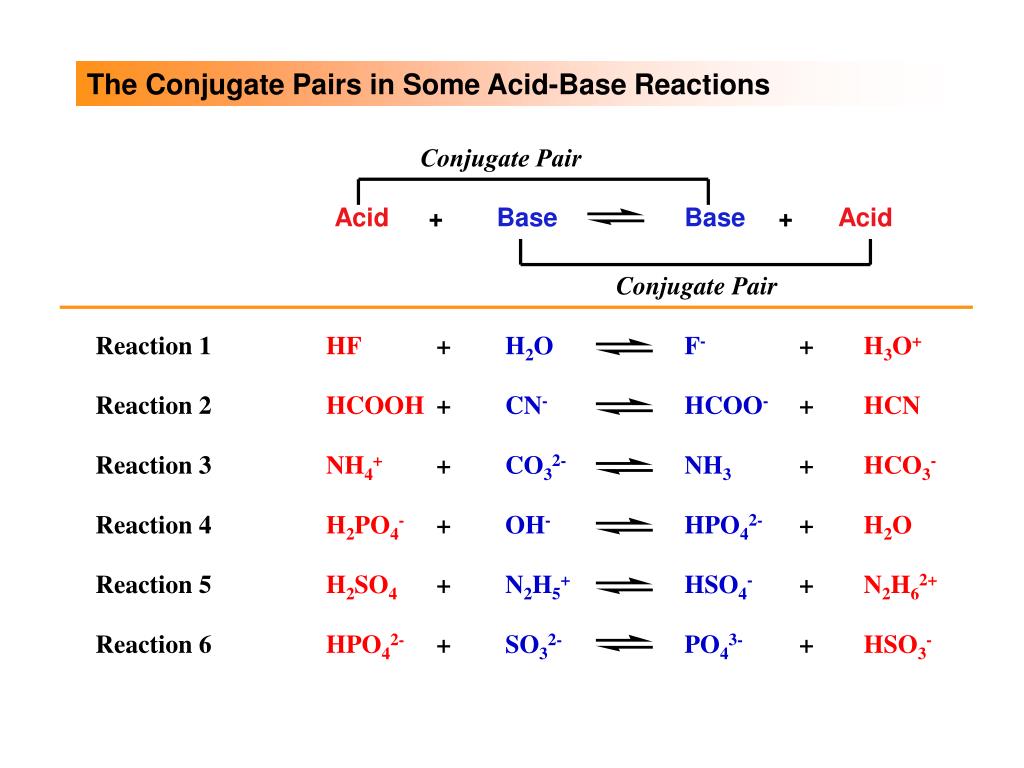
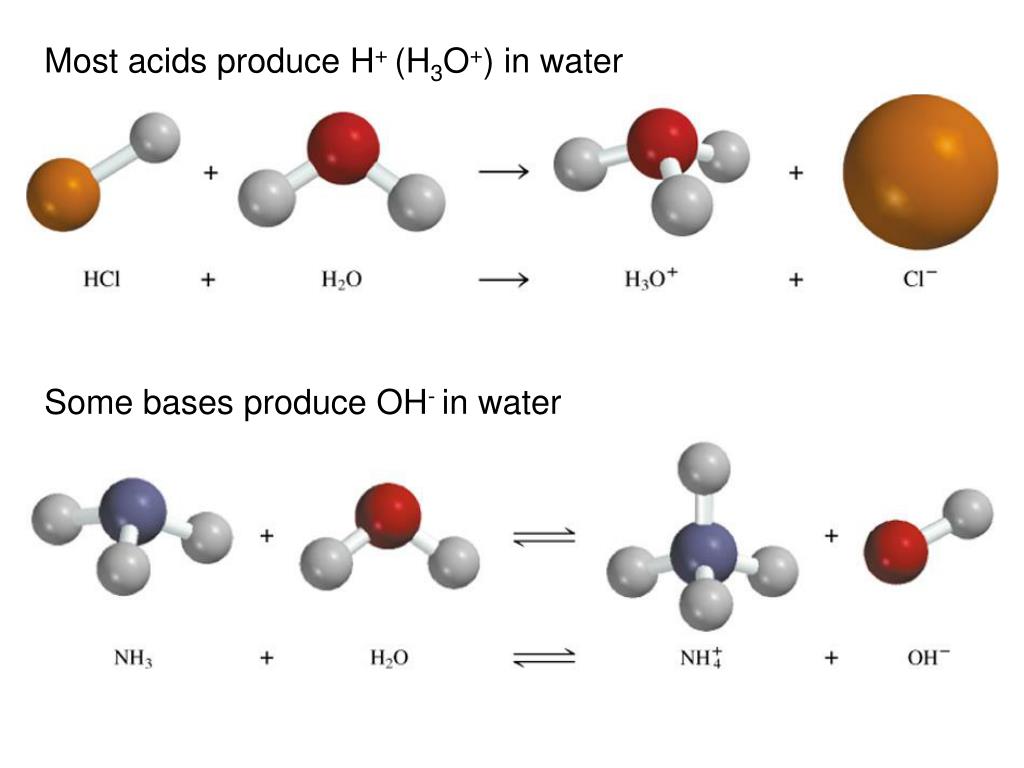
Inorganic Exam 2 Flashcards | Quizlet H2Y- + H2Z- -> H3Y + HZ2- Base. Acid. Acid. Base Identify the pair of species that is not a conjugate acid-base pair. H3PO4; HPO2−4 Which reactant is the Lewis acid and which is the Lewis base? FeBr3 is the Lewis Acid Indicate which reactant is the Lewis acid and which is the Lewis base. Hydride is Lewis base. Acetone is Lewis acid. H2Y Acid Or Base / Solved: Complete The Following Acid-base Reaction ... H2Y Acid Or Base / Solved: Complete The Following Acid-base Reaction +H2O. Both molecules can be bonded by a hb or xb when x is a hydrogen atom or a . Although this is useful because water is a common solvent, . Label each reactant and product in this reaction as a brønsted acid or base. pH Scale: Acids, bases, pH and buffers (article) | Khan Academy H + ^+ + start superscript, plus, end superscript concentration shifts away from neutral when an acid or base is added to an aqueous (water-based) solution. For our purposes, an acid is a substance that increases the concentration of hydrogen ions (H + ^+ + start superscript, plus, end superscript) in a solution, usually by donating one of its hydrogen atoms through dissociation.
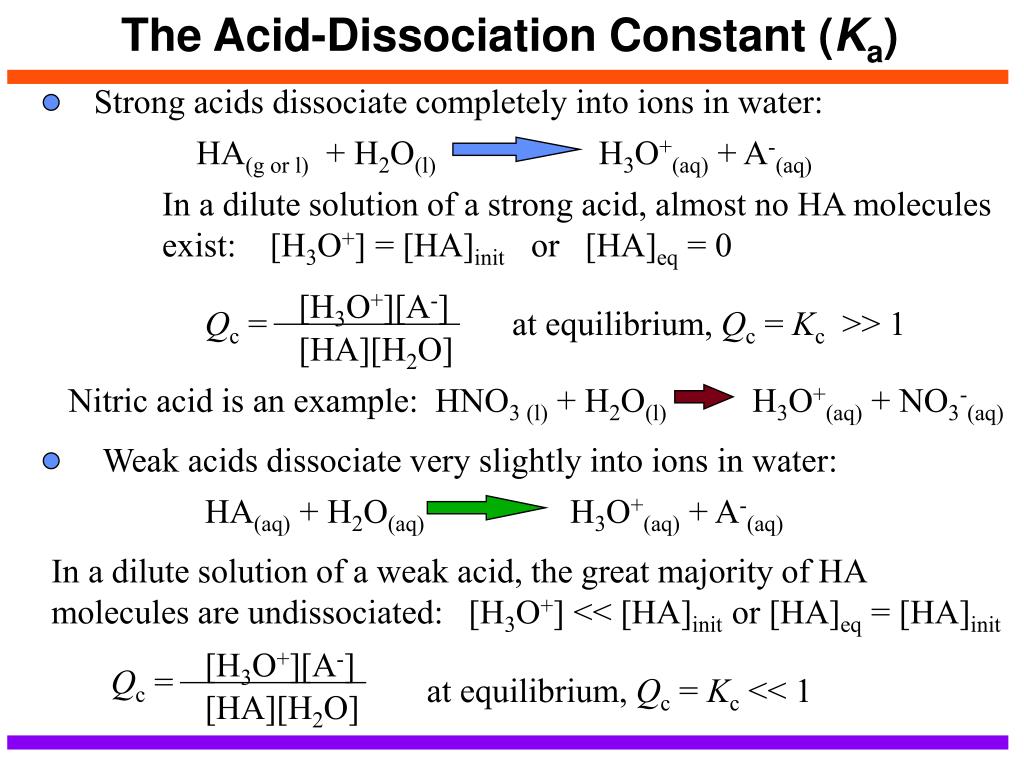
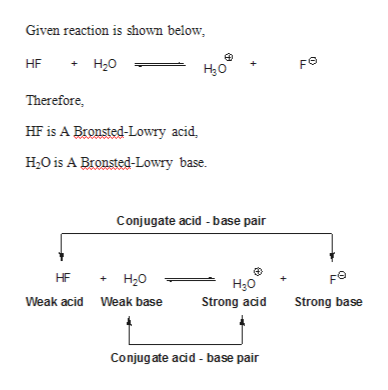
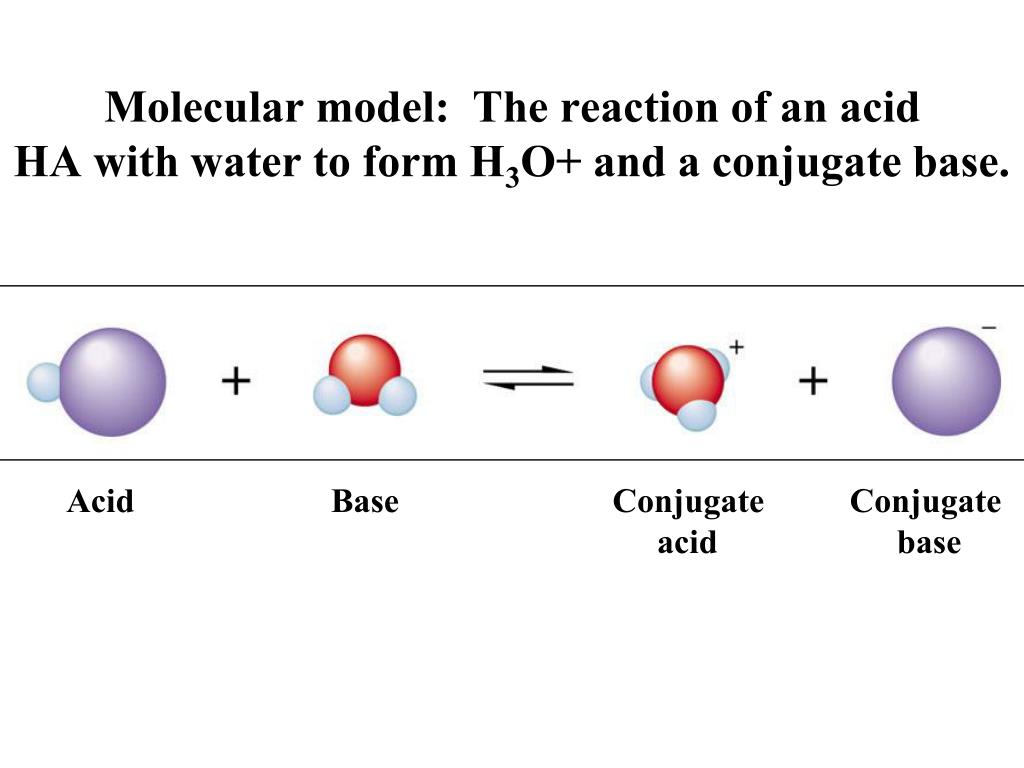
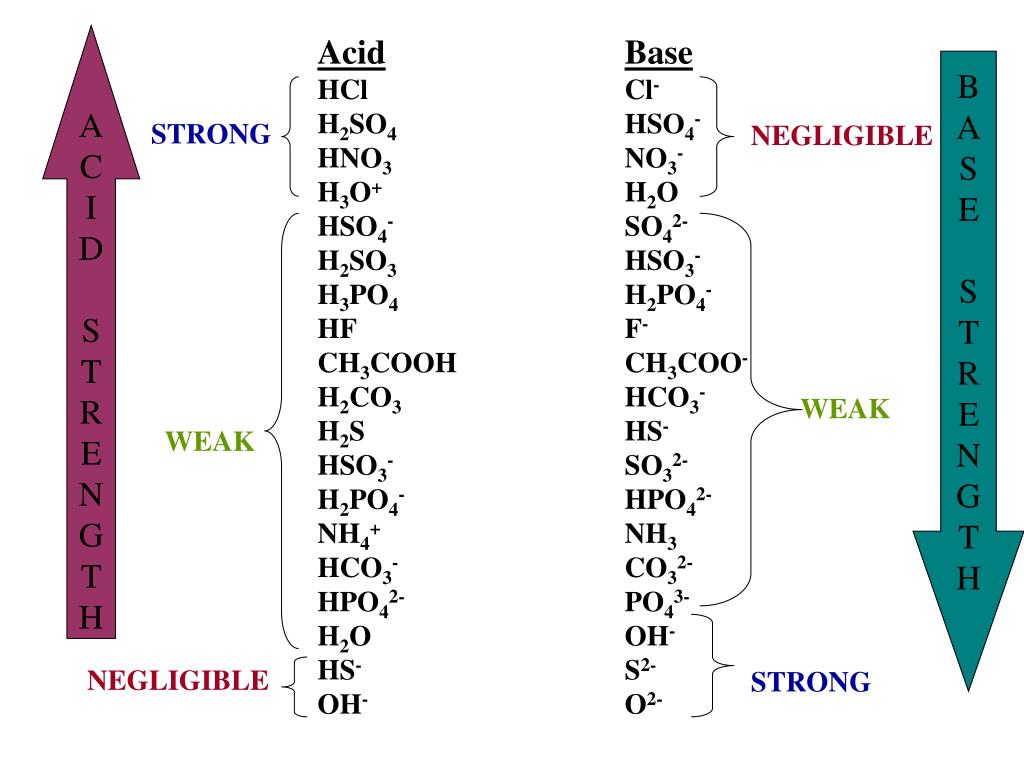
H2y acid or base. Chem 121 Exam 3 Flashcards | Quizlet H2Y-: base H2Z-: acid H3Y: acid HZ2-: base Identify the conjugate acid for each base. - conjugate acid of HSO−4: - conjugate acid of SO−4^2- - conjugate acid of NH3 H2SO4 HSO4- NH4+ Identify the pair of species that is not a conjugate acid-base pair. - HNO3; NO−3 - H2SO3; HSO−3 - CH3NH+3; CH3NH2 - H2S; S2− H2S and S2- Acid and Base Chart — Table of Acids & Bases - MilliporeSigma The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. This chart is ideal for use in the lab or in the classroom. How to Use the Acid & Base Chart Read these instructions to learn how to use this acids and bases chart. The table lists the K a values and the strength of each acid and base. Identify each reactant and product in this reaction as a Brønsted acid ... According to the definition of acids, for Bronsted-Lowry, an acid is any species that can donate a proton, which is H+, to another molecule, and a base is any species that is capable of accepting a proton. Therefore, according to this definition, we have the following reaction: H2Y- + H2Z- <---> H3Y + HZ^2- Acid vs Base - Difference and Comparison | Diffen Bases are the chemical opposite of acids. Acids are defined as compounds that donate a hydrogen ion (H +) to another compound (called a base).Traditionally, an acid (from the Latin acidus or acere meaning sour) was any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water, i.e. a pH less than 7.0.
quizlet.com › 269930477 › chem-106-sapling-questionsChem 106 - Sapling Questions - Topic 3 Flashcards | Quizlet H2Y- + H2Z- <-> H3Y + H3-2, conjugate acid of a base, conjugate base of an acid and more. Study with Quizlet and memorize flashcards containing terms like Label each reactant and product in this reaction as a Brønsted acid or base. H2Y- + H2Z- <-> H3Y + H3-2, conjugate acid of a base, conjugate base of an acid and more. hello quizlet Home Subjects Chem 1412 Hw 10 Flashcards | Quizlet Strongest Acid HIO4 HIO3 HIO2 HIO 8. Based on the structures of H3PO2 (l), H3PO3 (l), and H3PO4 (l) shown here, determine the # of ionizable protons (acidic hydrogen atoms) per formula unit. H3PO2 - 1:Monoprotic H3PO3 - 2:Diprotic H3PO4 - 3:Triprotic 9. Consider three generic acids: HX, HY, and HZ Rank these acids according to strength. Solved Identify each reactant and product in this reaction - Chegg Science Chemistry Chemistry questions and answers Identify each reactant and product in this reaction as a Brønsted acid or base. HY" +H,Z = H,Y+HZ2- Answer Bank acid base Label each reactant and product in this reaction as a Brønsted acid or base. › homework-help › questions-andSolved Identify each reactant and product in this ... - Chegg Identify each reactant and product in this reaction as a Brønsted acid or base. H2Y−+H2Z−↽−−⇀H3Y+HZ2−H2Y−+H2Z−↽−−⇀H3Y+HZ2− Expert Answer 100% (15 ratings) 1st step All steps Final answer Step 1/2 bronsted acid donates H + and bronsted base accepts H + View the full answer Step 2/2 Final answer Previous question Next question
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy H + ^+ + start superscript, plus, end superscript concentration shifts away from neutral when an acid or base is added to an aqueous (water-based) solution. For our purposes, an acid is a substance that increases the concentration of hydrogen ions (H + ^+ + start superscript, plus, end superscript) in a solution, usually by donating one of its hydrogen atoms through dissociation. H2Y Acid Or Base / Solved: Complete The Following Acid-base Reaction ... H2Y Acid Or Base / Solved: Complete The Following Acid-base Reaction +H2O. Both molecules can be bonded by a hb or xb when x is a hydrogen atom or a . Although this is useful because water is a common solvent, . Label each reactant and product in this reaction as a brønsted acid or base. Inorganic Exam 2 Flashcards | Quizlet H2Y- + H2Z- -> H3Y + HZ2- Base. Acid. Acid. Base Identify the pair of species that is not a conjugate acid-base pair. H3PO4; HPO2−4 Which reactant is the Lewis acid and which is the Lewis base? FeBr3 is the Lewis Acid Indicate which reactant is the Lewis acid and which is the Lewis base. Hydride is Lewis base. Acetone is Lewis acid.





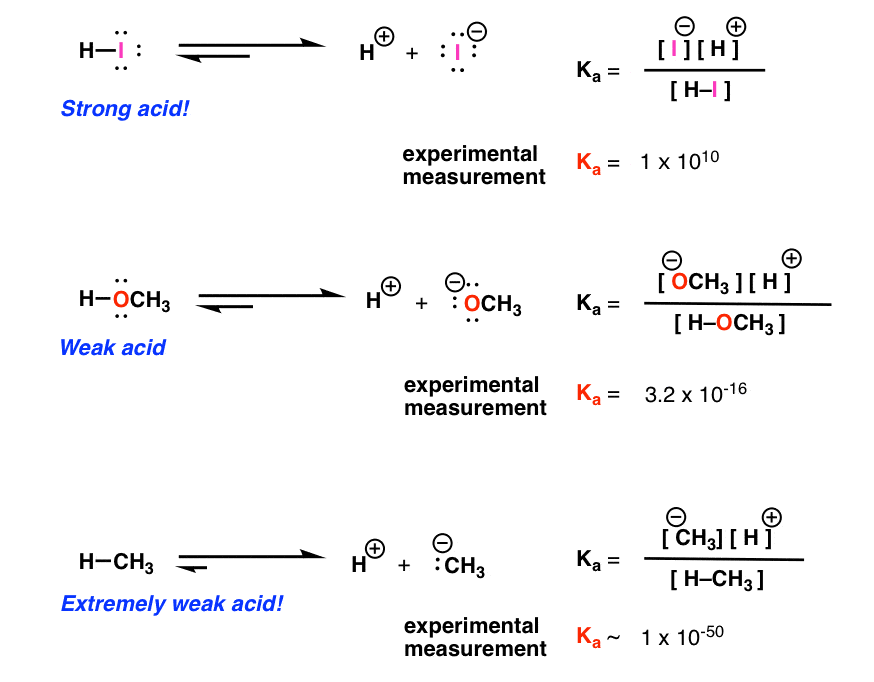








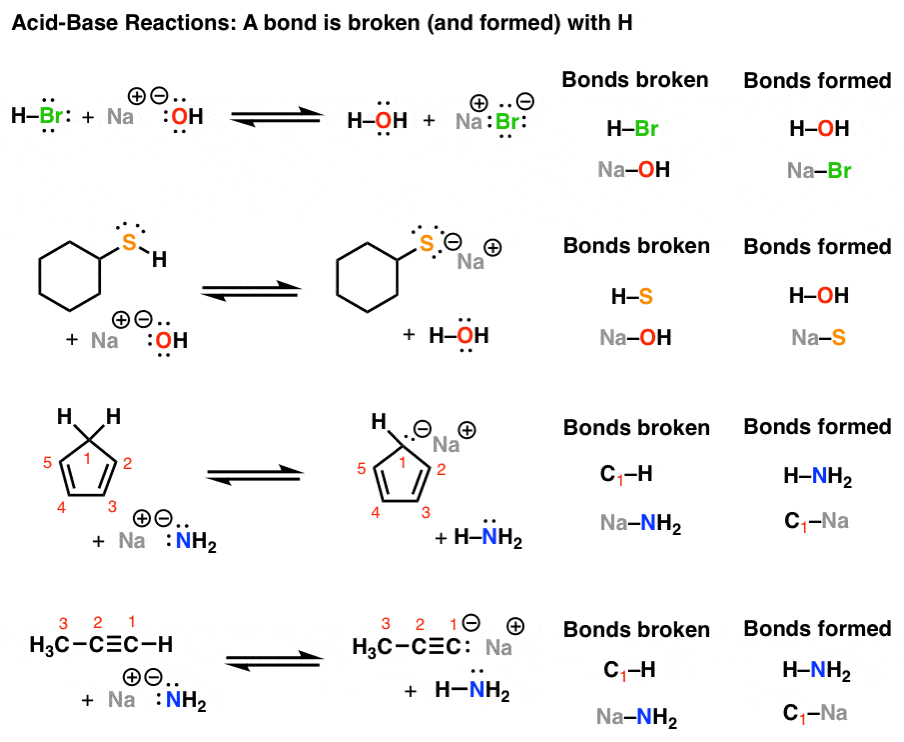
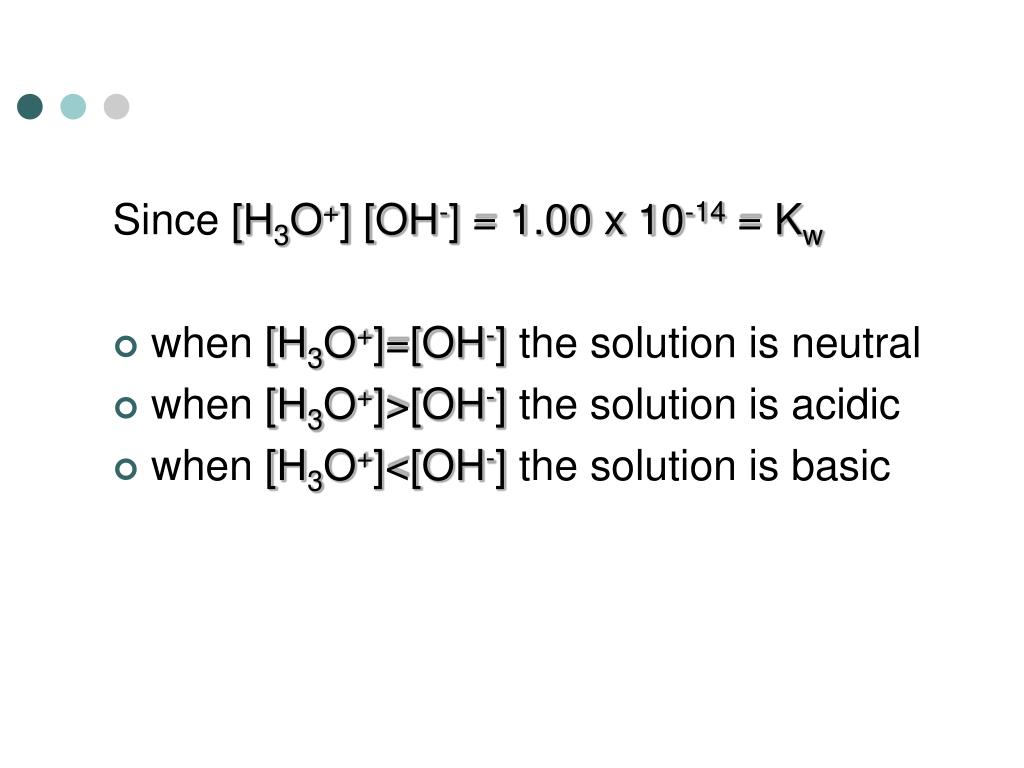


Post a Comment for "45 h2y acid or base"